|
|
Density
We have used a microfluidic mass sensor to measure the density of single living cells. By weighing each cell in two fluids of different densities, our technique measures the single-cell mass, volume, and density of ~500 cells per hour with a density precision of 0.001 g / mL. We observe that the intrinsic cell-to-cell variation in density is nearly 100-fold smaller than the mass or volume variation. As a result, we can measure changes in cell density indicative of cellular processes that would be otherwise undetectable by mass or volume measurements. Here we demonstrate this with four examples: identifying P. falciparum malaria-infected erythrocytes in a culture, distinguishing transfused blood cells from a patient’s own blood, identifying irreversibly-sickled cells in a sickle cell patient, and identifying leukemia cells in the early stages of responding to a drug treatment. These demonstrations suggest that the ability to measure single cell density will provide valuable insights into cell state for a wide range of biological processes. W.H. Grover, A.K Bryan, M. Diez-Silva, S. Suresh, J.M. Higgins, S.R. Manalis. Measuring single-cell density, Proceedings of the National Academy of Sciences (2011). Download PDF.See also MIT News. Deformability
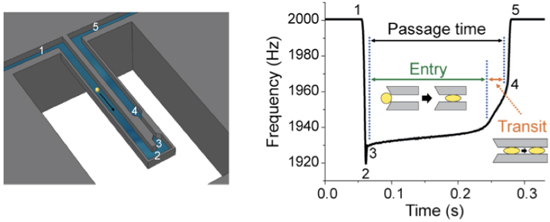
Metastasis requires the penetration of cancer cells through tight spaces, which is mediated by the physical properties of the cells as well as their interactions with the confined environment. Various microfluidic approaches have been devised to mimic traversal in vitro by measuring the time required for cells to pass through a constriction. Although a cell’s passage time is expected to depend on its deformability, measurements from existing approaches are confounded by a cell's size and its frictional properties with the channel wall. Here we introduce a device that enables the precise measurement of: i) the size of a single cell, given by its buoyant mass, ii) the velocity of the cell entering a constricted microchannel (entry velocity) and iii) the velocity of the cell as it transits through the constriction (transit velocity). Changing the deformability of the cell by perturbing its cytoskeleton primarily alters the entry velocity, whereas changing the surface friction by immobilizing positive charges on the constriction's walls primarily alters the transit velocity, indicating that these parameters can give insight into the factors affecting the passage of each cell. When accounting for cell buoyant mass, we find that cells possessing higher metastatic potential exhibit faster entry velocities than cells with lower metastatic potential. We additionally find that some cell types with higher metastatic potential exhibit greater than expected changes in transit velocities, suggesting that not only the increased deformability but reduced friction may be a factor in enabling invasive cancer cells to efficiently squeeze through tight spaces. S. Byun, S. Son, D. Amodei, N. Cermak, J. Shaw, J.H. Kang, V.C. Hecht, M.W. Winslow, T. Jacks, P. Mallick and S.R Manalis. Characterizing deformability and surface friction of cancer cells, Proceedings of the National Academy of Sciences (2013). Download PDF. See also MIT News. Dry mass, wet mass and dry density
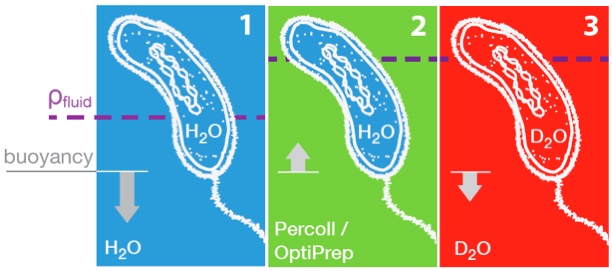
We developed a method for direct non-optical quantification of dry mass, dry density and water mass of single living cells in suspension. Dry mass and dry density are obtained simultaneously by measuring a cell’s buoyant mass sequentially in an H2O-based fluid and a D2O-based fluid. Rapid exchange of intracellular H2O for D2O renders the cell’s water content neutrally buoyant in both measurements, and thus the paired measurements yield the mass and density of the cell’s dry material alone. Utilizing this same property of rapid water exchange, we also demonstrate the quantification of intracellular water mass. In a population of E. coli, we paired these measurements to estimate the percent dry weight by mass and volume. We then focused on cellular dry density – the average density of all cellular biomolecules, weighted by their relative abundances. Given that densities vary across biomolecule types (RNA, DNA, protein), we investigated whether we could detect changes in biomolecular composition in bacteria, fungi, and mammalian cells. In E. coli, and S. cerevisiae, dry density increases from stationary to exponential phase, consistent with previously known increases in the RNA/protein ratio from up-regulated ribosome production. For mammalian cells, changes in growth conditions cause substantial shifts in dry density, suggesting concurrent changes in the protein, nucleic acid and lipid content of the cell. F.F. Delgado, N. Cermak, V.C. Hecht, S. Son, Y. Li, S.M. Knudsen, S. Olcum, J.M. Higgins, J. Chen, W.H. Grover, S.R. Manalis. Intracellular water exchange for measuring the dry mass, water mass and changes in chemical composition of living cells. PLoS ONE (2013). Download PDF. See also Suppl. Info.
|